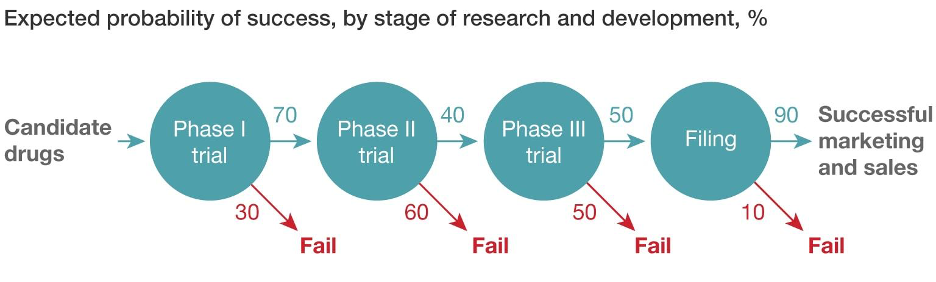
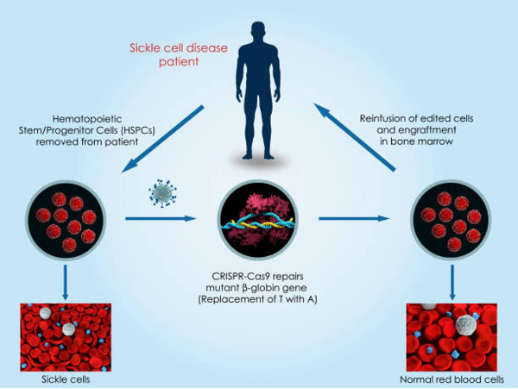
Communicating pharmaceutical safety and efficacy is a delicate and critical task, especially when discussing vaccines. These lifesaving interventions have faced increasing public skepticism, fueled by complex scientific information that is difficult to understand, compounded by societal mistrust arising from misinformation and falsified studies. Miscommunication or oversimplification can erode trust, while too much technical detail can alienate those who need a scientific background. How can we rebuild trust in vaccines and ensure the public understands their value?
At GreenField, one of the services we offer our clients is technical writing in support of their CMC submissions. The CMC is a highly technical document whose information is not readily assimilated by anyone outside the pharmaceutical industry. It’s meant to specifically communicate highly technical information from a qualified, educated, and experienced manufacturer to a qualified, educated, and experienced regulatory official. Although not publicly available, it serves as an example of the highly technical data that’s submitted in support of a drug application.
The Comprehension Gap
The disparity between the reading and comprehension levels of the average American and the technical complexity of vaccine and pharmaceutical drug product information is a key barrier. The average American reads at a 7th- to 8th-grade level (and a significant portion below the 6th-grade level), yet pharmaceutical information, including safety profiles and efficacy data, is often written at a college or postgraduate level.
For example:
- A drug product package insert might list rare adverse events using highly technical language like “thrombocytopenia” or “anaphylaxis,” terms that are unfamiliar and intimidating to most readers.
- Discussions of vaccine efficacy often involve statistical concepts like “relative risk reduction,” “confidence intervals,” or “number needed to vaccinate,” which can seem opaque without context.
These challenges are compounded when misinformation takes root. Misinterpretations of vaccine studies, such as the debunked 1998 paper falsely linking the MMR vaccine to autism, have had lasting impacts on public trust. Despite being retracted and widely discredited, this single falsified study has fueled a global movement of vaccine hesitancy and a resurgence in measles cases.
Mistrust of Vaccines: A Historical and Ongoing Challenge
Mistrust of vaccines often stems from misinformation amplified by social media, a lack of transparency in pharmaceutical communications, and the emotional resonance of personal anecdotes over scientific data. Examples include:
- The Wakefield Scandal: Andrew Wakefield’s fraudulent study falsely claiming a link between the MMR vaccine and autism has been repeatedly disproven, yet it ignited a wave of anti-vaccine sentiment.
- COVID-19 Vaccines: Despite their rapid development and lifesaving impact, misinformation about mRNA technology, fears of side effects, and political polarization created widespread hesitancy. This is despite the fact that mRNA technology was the culmination of 40 years of research with demonstrated applications pre-dating COVID by 10 years.
This mistrust is exacerbated by a lack of understanding about how vaccines work, their rigorous development processes, and their safety monitoring systems. For many, the idea of injecting a substance into the body without fully comprehending its mechanisms feels inherently risky. This mistrust in vaccines has carried over into mistrust of other pharmaceutical processes and emphasizes the challenges in relaying highly technical information to the general public.
Addressing the Gap and Rebuilding Trust
We must focus on education, transparency, and proactive communication to overcome these challenges. Here’s how:
1. Debunking misinformation
Efforts to counter misinformation must be timely, accessible, and widely disseminated. Clear, evidence-based rebuttals should address common myths. For example, Explaining why mRNA vaccines do not alter DNA can be paired with simple analogies, such as comparing mRNA to a “recipe” used by the body to make a harmless protein.
2. Transparency and Accountability
The pharmaceutical industry and regulatory agencies must be transparent about how vaccines are developed, tested, and monitored for safety. This includes explaining clinical trial phases and their role in ensuring vaccines are safe and effective. The public also needs to know how systems like the Vaccine Adverse Event Reporting System (VAERS) monitor rare side effects.
Transparency also means acknowledging and addressing past mistakes, like the Wakefield study, to demonstrate accountability.
3. Simplify the Science
Use plain language to explain key concepts. For instance, instead of saying “herd immunity,” explain that “vaccines protect not just individuals but entire communities, especially those who cannot be vaccinated, like newborns or people with weakened immune systems.” Also, writers need to bear the responsibility of avoiding technical jargon and focus on practical, relatable examples.
4. Engaging with Communities
Mistrust often stems from a need for more connection between experts and the communities they serve. Proactive engagement through town halls, social media Q&A sessions, and partnerships with trusted community leaders can foster understanding and trust. Faith leaders and local health advocates can be trained to discuss vaccine safety in culturally relevant ways. These outreach events need to extend beyond emergencies as they arise. Public engagement needs to be a 365-day-a-year activity
5. Providing Visual Aids
Graphics and animations can simplify complex ideas. Visualizing how vaccines stimulate the immune system or showing the real-world impact of vaccination programs can make data more relatable.
6. Educating About Risks in Context
People often fear rare side effects because they lack context. Explaining that “the chance of a serious allergic reaction to this vaccine is less than 1 in a million, while the chance of severe complications from the disease it prevents is much higher” helps to clarify relative risk.
Overcoming Societal Mistrust: The Role of Trustworthy Messengers
One of the most effective ways to rebuild trust in vaccines is through trusted messengers. These include healthcare providers, scientists, and even celebrities who can communicate the science in a relatable way. Messages must be empathetic and acknowledge people’s concerns without dismissing them. They need to avoid condescension or over-complication. And, they need to reaffirm the shared goal of protecting health and saving lives.
Conclusion
Bridging the gap between technical vaccine/pharmaceutical drug product information and public comprehension is about more than simplifying science. It’s about rebuilding trust in the systems that protect public health. By focusing on transparency, clarity, and engagement, we can address fears, counter misinformation, and empower individuals to make informed decisions about their health. Vaccines are one of the most significant achievements in medical history, and ensuring public trust in them is essential to realizing their full potential. The one thing that we cannot do is allow quackery and debunked pseudoscience to gain a toehold. The Wakefield study continues to cause problems nearly 30 years after its publication and debunking.