Sickle cell disease (SCD), a genetic and inheritable blood disorder characterized by sickle-shaped red blood cells, impacts approximately 100,000 patients in the US and over 20 million people worldwide. The emergence of CASGEVY™ (exagamglogene autotemcel), the first CRISPR-Cas9-based gene therapy approved for SCD, provides hope for those afflicted with this disorder.
Traditionally, SCD was treated through blood transfusions, bone marrow transplants, and supportive measures (hydroxyurea, OTC NSAIDs, voxelotor, etc.) offering limited relief from chronic pain, fatigue, and organ damage. The quest for a definitive cure led to explorations of gene therapy, initially utilizing lentiviral vectors for β-globin gene correction. However, concerns about insertional mutagenesis prompted a shift towards the precision of CRISPR-Cas9 technology.
Vertex Pharmaceuticals and CRISPR Therapeutics spearheaded the development of CASGEVY™, employing CRISPR-Cas9’s unparalleled accuracy and efficiency. Pre-clinical studies in human hematopoietic stem cells established successful β-globin gene modification, paving the way for clinical trials. These pivotal trials, HITI-186 and HITI-229, provided hope for patients waiting for a permanent solution.
Patients underwent leukapheresis to collect their hematopoietic stem cells, subsequently edited ex vivo using CRISPR-Cas9 to introduce a functional β-globin gene. Reinfusion of these modified cells held the promise of producing healthy red blood cells. The results were transformative, with both trials demonstrating significant reductions in vaso-occlusive crises (VOCs), the hallmark pain episodes of SCD, alongside substantial improvements in patient-reported outcomes (PROs). This resonated deeply within the SCD community, generating widespread anticipation for CASGEVY™’s broader application.
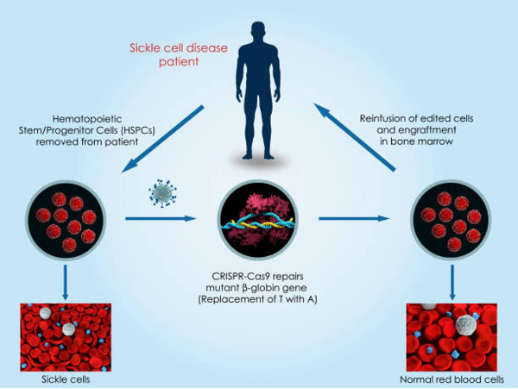
Figure 1: Approaches to CRISPR-Cas9 mediated SCD Gene Therapy (https://www.synthego.com/crispr-sickle-cell-disease)
The path to regulatory approval was complex yet ultimately successful. In December 2023, CASGEVY secured the coveted status of the first FDA-approved CRISPR-based gene therapy, marking a historical turning point in both gene therapy and SCD treatment. Conditional approval by the EMA in November 2023 further solidified its potential, with final authorization eagerly awaited. With its indication restricted to individuals aged 12 and above with recurrent VOCs, CASGEVY™ represents a targeted yet crucial first step towards transforming the lives of countless individuals burdened by SCD.
While CASGEVY™’s approval is a monumental triumph, challenges remain. Long-term safety and efficacy data necessitate continued monitoring and the potential for off-target effects of CRISPR editing demands further investigation. Additionally, the intricate manufacturing process and high cost (projected at $2 million per patient) of CASGEVY pose potential barriers to access for many patients. Addressing these challenges through ongoing research, cost-reduction strategies, and broadened healthcare policies is crucial to ensure this life-altering therapy reaches those most in need.
CASGEVY™ is more than just a gene therapy; it is a beacon of hope for the SCD community. It illuminates a future where genetic modifications may permanently alleviate the suffering caused by SCD, enabling individuals to flourish instead of endure. Continued research, dedicated efforts to ensure accessibility, and unwavering scientific optimism are essential to realizing the transformative potential of CASGEVY™ fully. With each advancement, the shadows cast by SCD will recede further, allowing the light of hope to illuminate a brighter future for those living with this challenging condition.
For more information on this novel therapy, see:
- https://www.businesswire.com/news/home/20231208951733/en/
- https://time.com/6343853/fda-crispr-treatment-sickle-cell/
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
- https://www.sicklecelldisease.org/2023/10/26/scdaa-statement-on-exa-cel-gene-therapy/
- https://www.scientificamerican.com/article/fda-approves-first-crispr-gene-editing-treatment-for-sickle-cell-disease/
- https://www.oligotherapeutics.org/exa-cel-a-potential-breakthrough-with-astounding-results/
