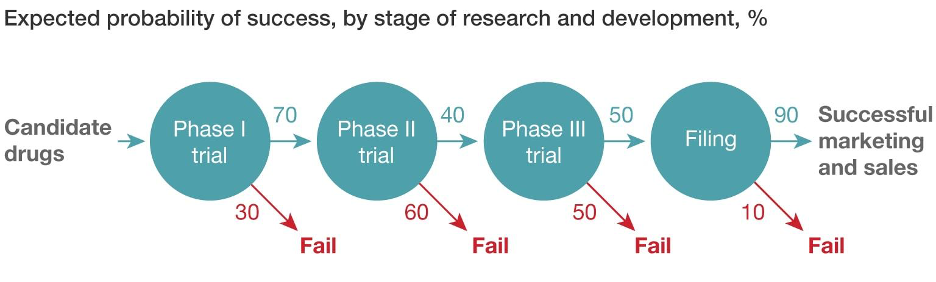
The journey from a promising molecule to a life-saving medication is complex. Drug development is a marathon, not a sprint. There are scientific uncertainties, regulatory hurdles, and ever-present financial pressures. Bringing a single drug to market can take over a decade and cost billions of dollars, with only a 10% success rate (references 1 and 2). So, how do pharmaceutical companies navigate this intricate maze and increase their chances of success?
Two powerful tools help manage drug development: the Stage-Gate Process and Integrated Risk Management (IRM). The Stage-Gate Process breaks development into phases with clear goals and decision points, while IRM identifies and mitigates risks throughout. Together, they improve efficiency, minimize risks, and increase the chances of delivering effective treatments.
The Stage-Gate Process
The Stage-Gate Process is a structured project roadmap that divides drug development into stages, each with specific goals and decision points to foster efficiency and ensure informed decision-making.
Stage 1: Scoping and Feasibility
In this stage, the basics of the drug development project are defined. Key questions addressed include:
- Defining the drug product and desired dosage form.
- Assessing manufacturing feasibility.
- Evaluating potential CMC risks (e.g., raw material availability, scalability).
- Developing a preliminary CMC development plan and budget.
A feasibility study evaluates scientific, technical, regulatory, and market feasibility. Based on this, a Go/No-Go decision is made to proceed with development or reconsider the project.
Stage 2: Preclinical Development
This stage focuses on testing the drug’s safety and efficacy in animals. CMC activities include:
- Developing analytical methods to ensure consistency and quality.
- Formulation optimization for stability and delivery.
- Supporting toxicity studies with CMC data.
A Go/No-Go decision is made based on preclinical data and CMC’s contribution to refine or continue development.
Stage 3: Clinical Development
In this stage, the drug is tested in human trials. CMC activities include:
- Scaling up manufacturing processes.
- Producing clinical trial materials under GMP guidelines.
- Conducting stability studies to determine shelf life and storage conditions.
CMC collaborates with clinical teams to ensure quality and compliance, with a Go/No-Go decision based on trial data.
Stage 4: Regulatory Approval
CMC plays a critical role in assembling the CMC package for regulatory agencies, including:
- Detailed manufacturing process descriptions.
- Analytical methods for quality assurance.
- Product characterization and stability studies.
- Quality control procedures.
Successful CMC strategies increase the chances of regulatory approval.
Stage 5: Launch and Post-Marketing Surveillance
With regulatory approval, CMC focuses on:
- Transferring manufacturing processes to commercial sites.
- Implementing quality management systems.
- Monitoring product quality and investigating adverse events.
- Continuous improvement of manufacturing processes and drug products.
CMC ensures ongoing safety and efficacy of the drug post-market.
Conclusion: The Advantages of Stage-Gate for CMC Management
The Stage-Gate Process enhances efficiency, mitigates risks, supports data-driven decision-making, and ensures seamless integration with overall development. It accelerates time to market and improves regulatory compliance, offering a flexible framework for successful drug development. By employing the Stage-Gate Process, CMC teams can navigate drug development more efficiently and confidently, ensuring the delivery of safe and effective medications to patients.
